Where Do You Start Iv Distal or Proximal if You Miss and Need to Try Again
Chapter 8. Intravenous Therapy
8.4 Priming IV Tubing and Changing Four Fluids and Tubing
Primary and secondary IV tubing and add-on devices (extension tubing) must exist primed with 4 solution to remove air from the tubing. Priming refers to placing IV fluid in IV tubing to remove all air prior to attaching the Four tube to the patient. IV tubing is primed to preclude air from entering the circulatory system. An air embolism is a potential complexity of Four therapy and can enter a patient'southward blood system through cutting tubing, unprimed IV tubing, access ports, and drip chambers with too niggling fluid (Perry et al., 2014). Information technology is unknown how much air will cause death, just deaths take been reported with as fiddling as 10 ml of air. The all-time fashion to avoid air bubbling in Four tubing is to forbid them in the first identify (Perry et al., 2014). New 4 tubing may also be required if leaking occurs around the tube connecting to the IV solution, if the tubing becomes damaged, or if it becomes contaminated. Checklist 66 outlines the process of priming IV tubing.
Disclaimer: Always review and follow your infirmary policy regarding this specific skill. | ||||
Safety considerations:
| ||||
Steps | Additional Information | |||
| one. Perform hand hygiene. | This footstep prevents the transmission of microorganisms. | |||
| ii. Check order to verify solution, rate, and frequency. | This ensures IV solution is correct and helps prevent medication error. | |||
| three. Gather supplies. | You volition need IV solution, primary IV tubing, fourth dimension label, change label, alcohol swab, and bowl or sink. 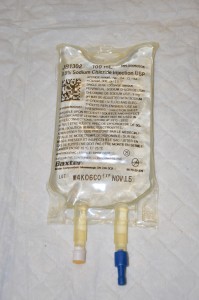 | |||
| 4. Remove IV solution from outer packaging and gently squeeze. | Tear the perforated corner of the outer packaging; check colour, clarity, and expiration date.  | |||
| five. Remove primary IV tubing from outer packaging. |  | |||
| 6. Move the roller clamp about 3 cm below the drip chamber and shut the clamp. | 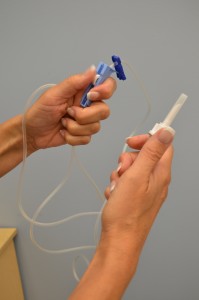 | |||
| vii. Remove the protective cover on the IV solution port and go along sterile. Remove the protective cover on the Iv tubing spike. | Exist conscientious and practise not contaminate the spike.  | |||
| 8. Without contaminating the solution port, carefully insert the IV tubing spike into the port, gently pushing and twisting. | 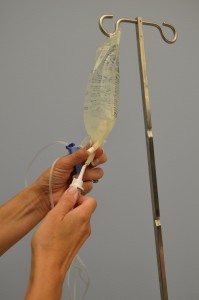 | |||
| 9. Hang bag on Four pole. | The IV bag should be approximately one metre above the IV insertion site. | |||
| 10. Fill the drip bedroom one-third to 1-half full by gently squeezing the sleeping accommodation. Remove protective cover on the end of the tubing and keep sterile. | Filling the drip sleeping accommodation prevents air from entering the 4 tubing. 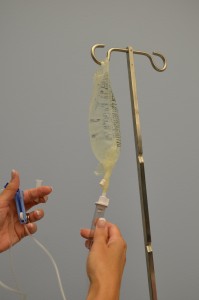 | |||
| 11. With distal cease of tubing over a basin or sink, slowly open roller clench to prime the IV tubing. Capsize backcheck valve and ports as the fluid passes through the tubing. Tap gently to remove air and to fill with fluid. | Inverting and tapping the access ports and backcheck valve helps readapt and remove air when priming the IV tubing. 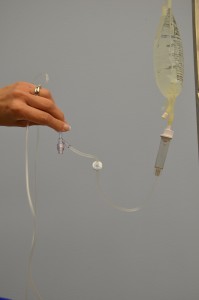 | |||
| 12. Once IV tubing is primed, bank check the entire length of tubing to ensure no air bubbles are present. | This stride confirms that air is out of the IV tubing. | |||
| 13. Shut roller clamp. Cover cease with sterile dead-ender or sterile protective cover. Hang tubing on Iv pole to forestall from touching the ground. | Keep the distal terminate sterile prior to connecting 4 to patient. | |||
| 14. Characterization tubing and 4 bag with date, fourth dimension, and initials. | Label Four solution pocketbook equally per agency policy. Do non write directly on the IV bag. 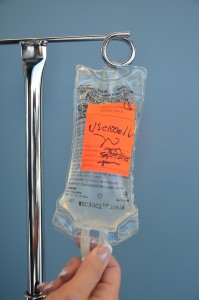 | |||
| xv. Perform paw hygiene. | This reduces the transmission of microorganisms.  | |||
| Data source: Fulcher & Frazier, 2007; Perry et al., 2014. | ||||
Video 8.1
Scout the videoPriming IV Lines by Renée Anderson & Wendy McKenzie, Thompson Rivers University.
4 solutions are considered sterile for 24 hours. An IV solution may exist changed if the dr.'s order changes, if an IV solution infusing at TKVO is expired afterward 24 hours, or if the IV solution becomes contaminated. To alter an IV solution bag, follow Checklist 67.
Disclaimer: Always review and follow your hospital policy regarding this specific skill. | ||||
Steps | Additional Information | |||
| 1. Verify and select right Four solution bag and compare to the medication administration record (MAR) or physician orders. | IV solutions are considered a medication and must exist checked using the SEVEN RIGHTS x 3, as per agency policy.  | |||
| 2. Introduce yourself, identify patient, and explain procedure. | Proper identification of a patient prevents medication errors. Explaining the procedure provides an opportunity for the patient to ask questions. | |||
| 3. Perform hand hygiene. | Hand hygiene prevents the manual of microorganisms. | |||
| 4. Remove outer plastic packaging and squeeze pocketbook to test for leaks and expiration engagement. Assess for precipitates or cloudiness. Hang new Iv solution on Iv pole. | This ensures the correct IV solution is used.  | |||
| five. Pause the EID or close the roller clamp on a gravity infusion set. | Stops the infusion to forbid air bubbles from forming in 4 tubing. | |||
| half dozen. Remove protective plastic embrace from the new Iv solution tubing port. | Go along Four tubing port sterile at all times. If 4 tubing port becomes contaminated, dispose of it immediately and replace. | |||
| 7. Remove the old Four solution bag from the Four pole. Turn Four bag upside down, grasping the tubing port. With a twisting motion, carefully remove IV tubing spike from old IV solution bag. | Removing sometime solution from Four pole prevents spilling of solution. Ensure IV tubing spike remains sterile during removal to avert contaminating IV tubing. | |||
| viii. Using a gentle twisting motion, firmly insert the fasten into the new Iv purse. | This ensures that a sterile technique is used during the process. 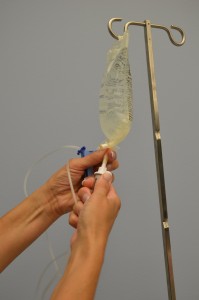 | |||
| nine. Fill the drip chamber past compressing information technology between your thumb and forefinger. Ensure the drip bedchamber is one-third to half full. Check IV tubing for air bubbling. | Fluid in the drip chamber helps forbid air from existence introduced into Four tubing. 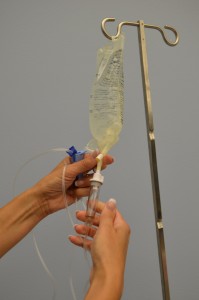  | |||
| 10. Open clamp and regulate IV infusion rate via gravity, or press outset on the EID as per md orders. | Once rate is fix, count the drops per minute on the gravity ready or ensure the EID is running at the correct rate as per medico orders.  | |||
| eleven. Label new IV solution bag equally per agency policy. Time tape gravity IV solutions equally per agency policy | Labelling IV solutions provides easy viewing of infusing solutions and additives.  | |||
| 12. Dispose of used supplies, perform paw hygiene, and document IV solution handbag change according to agency policy. | Certificate time, date, type of solution, rate, and full volume. | |||
| Data source: Fulcher & Frazier, 2007; Perry et al., 2014. | ||||
Video viii.2
Watch the video Changing IV numberlessby Renée Anderson & Wendy McKenzie, Thompson Rivers University.
Checklist 68 describes how to alter the Iv tubing administration set and IV solution at the aforementioned time.
Disclaimer: Always review and follow your hospital policy regarding this specific skill. | ||||
Steps | Additional Information | |||
| 1. Verify dr. orders for the type of solution, rate, and duration. Collect necessary supplies. | This step verifies the patient'south need for Four fluids/medications. It as well confirms the right rate and solution for patient safety. | |||
| 2. Perform manus hygiene. | Mitt hygiene prevents the transmission of microorganisms. | |||
| 3. Identify yourself, identify the patient using ii identifiers, and explain the procedure to the patient. | Proper identification of patient prevents errors.  | |||
| 4. Prime new assistants set using a new IV solution bag and new IV tubing. | 4 solutions are considered a medication. Prime every bit per Checklist 66. Keep distal protective cap attached to Four tubing to ensure sterility of distal stop. Label IV solution and Iv tubing as per agency policy.  | |||
| 5. Hang new assistants set (primed primary line and Four solution) on 4 pole. | This prepares the equipment and adheres to the principles of aseptic technique. | |||
| 6. Clamp quondam 4 administration set up. Remove IV tubing if on an EID. | Stop the flow of infusion during tubing and solution change. | |||
| vii. Make clean the connectedness between the distal end of old IV tubing and the positive force per unit area cap. Scrub the surface area for 15 seconds and let information technology dry for thirty seconds. | Proper disinfection of equipment decreases bacterial load and prevents infections. 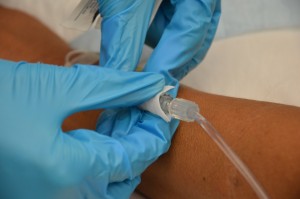 | |||
| 8. Remove the protective cap on the distal end of the new Iv administration set. |  | |||
| 9. Carefully disconnect the quondam tubing from the positive pressure level cap and insert the new IV tubing into the positive pressure cap fastened to the extension tubing. | 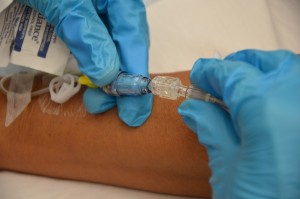 | |||
| ten. Open the roller clamp on the new tubing to regulate catamenia charge per unit, or insert new tubing into the EID and restart 4 rate. | This stride ensures the Iv solution is infusing at the right rate.  | |||
| 11. Check IV site for patency, and signs and symptoms of phlebitis. | Four site should be free from redness, swelling, and pain. Dressing on IV site should be dry and intact. 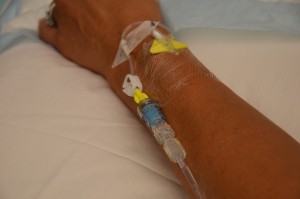 | |||
| 12. Discard onetime supplies and perform mitt hygiene. | This stride prevents the spread of microorganisms.  | |||
| xiii. Document procedure as per agency policy. | Document the date and time of 4 tubing and solution change. | |||
| Data source: BCIT, 2015b; Fulcher & Frazier, 2007; Perry et al., 2014 | ||||
- How long tin can 4 solution exist used?
- What is the purpose of removing air from 4 tubing?
Source: https://opentextbc.ca/clinicalskills/chapter/8-4-iv-assessment-maintenance-troubleshooting-and-discontinuation/
0 Response to "Where Do You Start Iv Distal or Proximal if You Miss and Need to Try Again"
Post a Comment